Factory facilities
In order to manufacture safe and high quality products based on our cultivation technology cultivated for over 50 years, we manufacture our products in a hygienic environment and management methods in accordance with Good Manufacturing Practice (GMP) for dietary supplements, including a series of manufacturing processes and quality inspections, to ensure the safety of our products. We strive to ensure that our products are safe and effective.
We also obtained FDA registration certification in December 2021.
Factory facilities
In order to manufacture safe and high quality products based on our cultivation technology cultivated for over 50 years, we manufacture our products in a hygienic environment and management methods in accordance with Good Manufacturing Practice (GMP) for dietary supplements, including a series of manufacturing processes and quality inspections, to ensure the safety of our products. We strive to ensure that our products are safe and effective.
We also obtained FDA registration certification in December 2021.
Plant No.1
We manufacture all processes from the extraction facilities for shiitake mushroom mycelial culture medium extract [LEM] and mannentake mushroom mycelial culture medium extract [MAK] to the commercialization of these products at our first factory. The highly confidential plant and aseptic facilities provide protection. Integrated production system with programmed computers. In a hygienic environment, production is carried out smoothly from cultivation to extraction, and from filling to packaging.

▒ Large extraction tanks
In the production of LEM [shiitake mushroom mycelia culture medium extract] and MAK [Mannentake mushroom (Ganoderma lucidum) mycelia culture medium extract], thorough control is required for production due to the long incubation period of solid culture medium. Noda Shokubutsu Kogyo has made large tank extraction of solid culture medium possible with its unique know-how cultivated up to now. We also maintain sanitary conditions in the work area in a clean room.
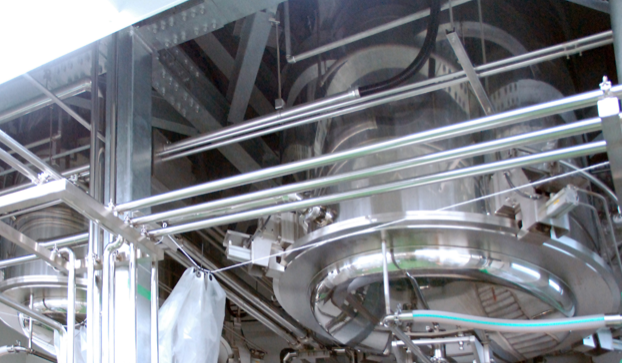
No.2 Plant
To be in harmony with nature and to be on the cutting edge. That is the product policy of Noda Shokubutsu Kogyo Co., Ltd. for natural health foods of natural origin.
In the second factory, we produce shiitake mushroom rice bran fermentation extract [LEF] by liquid culture.
It is also equipped with facilities for the production of powder products.

▒ Large liquid culture tank
To be in harmony with nature and to be on the cutting edge. That is the product policy of Noda Shokubutsu Kogyo Co., Ltd. for natural health foods of natural origin.
The second production plant produces shiitake rice bran fermentation extract [LEF] by liquid culture. LEF is a fermented food extracted from pre-treated rice bran with a culture of shiitake mushroom fungus.
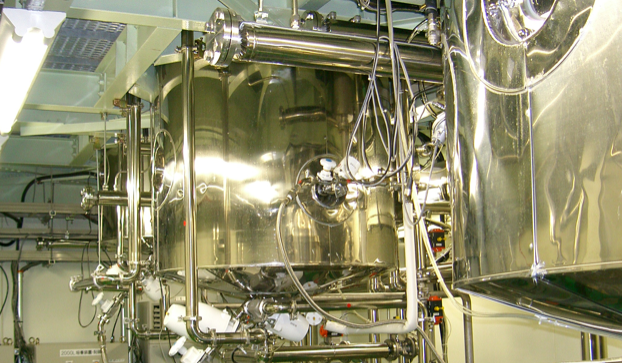
Plant No. 3
Noda Shokukin Kogyo has made solid culture possible through its unique know-how cultivated over the years. From seed culture to inoculation. Because of the long incubation period of solid culture media, thorough control of room temperature, humidity, and light is required for production.

Research Facilities
[Research Office/Quality Control Office]
In order to maintain a high level of quality in terms of product safety, stability, and efficacy, we strictly check the raw materials we use upon acceptance after collecting information on their origin, place of origin, manufacturing method, and quality standards.
For our own products, we adhere to design and compliance backed by scientific evidence. In order to strengthen our R&D capabilities, we have introduced advanced analytical equipment and manufacturing facilities for trial manufacturing experiments to promote efficient R&D of our own products.

